
There may be variations in CSA schedules between individual states. The Controlled Substances Act (CSA) schedule information displayed applies to substances regulated under federal law. The following drugs are listed as Schedule 4 (IV) Drugs* by the Controlled Substances Act (CSA): Abuse of the drug may lead to limited physical dependence or psychological dependence relative to the drugs in schedule 3. Solubility Enhancement of BCS Classified II/IV Drug Solid Dispersion of Apixaban by Solvent Evaporation. BCS Class 2 Drugs List BCS Class 3 Drugs List 0 Comments. A drug substance is considered highly permeable when the extent of absorption in humans is determined to be 90% or more of the administered dose based on a mass-balance determination or in comparison to an intravenous dose.įor dissolution class boundaries, an immediate release product is considered rapidly dissolving when no less than 85% of the labeled amount of the drug substance dissolves within 15 minutes using USP Dissolution Apparatus 1 at 100 RPM or Apparatus 2 at 50 RPM in a volume of 900 ml or less in the following media: 0.1 M HCl or simulated gastric fluid or pH 4.5 buffer and pH 6.8 buffer or simulated intestinal fluid.The drug has a low potential for abuse relative to the drugs in schedule 3 The drug has a currently accepted medical use in treatment in the United States. Asati A, Salunkhe K, Chavan M, Chintamani R, Singh R. Alternatively non-human systems capable of predicting drug absorption in humans can be used (such as in-vitro culture methods). Permeability class boundaries are based indirectly on the extent of absorption of a drug substance in humans and directly on the measurement of rates of mass transfer across human intestinal membrane. The volume estimate of 250 ml is derived from typical bioequivalence study protocols that prescribe administration of a drug product to fasting human volunteers with a glass of water. bioequivalence of two drug products 4, 5. After reviewing 130 drugs listed in the WHO Essential Medicines List regarding their. type of dissolution media for various BCS class drugs, their importance & methodology of dissolution. A drug is considered highly soluble when the highest dose strength is soluble in 250 ml or less of aqueous media over the pH range of 1 to 7.5. Biopharmaceutical drug disposition classification system. Solubility class boundaries are based on the highest dose strength of an immediate release product. Furthermore, a newly modified-version of BCS, biop-harmaceutics drug disposition classification system (BDDCS), which classify drugs into four categories according to sol-ubility and metabolism, has been introduced and gained much attention as a new insight in respect with the drug classification. The drugs are classified in BCS on the basis of solubility, permeability, and dissolution. Usually they are not well absorbed over the intestinal mucosa and a high variability is expected. For instance, products containing BCS class IV APIs are excluded from the BCS.

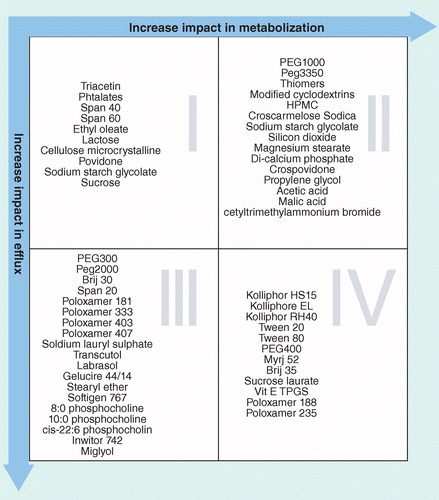

Those compounds are well absorbed and their absorption rate is usually higher than excretion.Class I - high permeability, high solubility.According to the Biopharmaceutical Classification System (BCS) drug substances are classified to four classes upon their solubility and permeability:


 0 kommentar(er)
0 kommentar(er)
